afrin nasal spray pregnant
 Afrin During Pregnancy: Is It Safe?
Afrin During Pregnancy: Is It Safe?Warning: The NCBI website requires JavaScript to operate. Use of desenganches during pregnancy and the risk of birth defects Abbreviations: BDS, Slone Epidemiology Center Birth defects study; CI, confidence interval; OR, probability ratio; OTC, on accountant. Associated data Abstract Previous studies suggested that early pregnancy exposure to certain oral decongestors increases the risks of several birth defects. Using data from January 1993 to January 2010 from the Slone Epidemiology Center Study of birth defects, we test these hypothesis between 12,734 babies with malformations (cases) and 7,606 un malformed control babies in the United States and Canada. Adjusted probability rates and 95% confidence intervals were calculated for specific birth defects, with control for potential confusers. The findings did not replicate several hypotheses, but they did support 3 previously reported associations: phenylephrine and endocardial damping (proportion of the diodes = 8.0; 95% confidence interval: 2.5, 25.3; 4 exposed cases), phenylpropanolamine and ear defects (proportion of the diodes = 7.8; 95% confidence interval: 2.2, 27.2; 4 cases Analysis generated by hypothesis involving multiple comparisons identified a small number of associations with oral and intranasal decongestants. The accumulation of tests supports the associations between the use of first quarters of certain oral and possibly intranasal decongestives and the risk of some specific rare birth defects. Decreases, in particular pseudoephedrine, are among the most used free-sale (OTC) medicines during pregnancy (). Because they are available without a prescription, they are widely perceived as insurance by health care providers and pregnant women, however the safety of prenatal exposure with respect to specific birth defects remains unclear. Given its wide use, even a small increase in the risk of birth defects would have significant implications for public health. All decongestors are vasoconstrictive and can share class-related effects. On the other hand, teratogenicity may vary within a particular class (), which makes it necessary to consider the specific decongestants as well. Specific epidemiological studies of congestors have identified high risks of specific birth defects, including heart defects, eyes and ears, intestine, abdominal wall, and feet (available in ) (–). Using data from the Slone Epidemiology Center Study of Birth Defects (BDS), we tested previously reported associations between specific birth defects and first-quarter exposure to oral decongestants and, in hypothesis-generation analysis, we explore the risks of other common congenital malformations. In addition, we explore the risk of specific birth defects in relation to the use of intranasal decongestants in the first quarter. MATERIALS AND POPULATIONS The BDS was initiated in 1976 as a programme to monitor cases of birth defects in relation to medicines. The details of the methods have been described (, ). The present analysis was based on data on topics interviewed between January 1993 and January 2010. During those years, study nurses interviewed women within 6 months of childbirth who had identified malformed babies or fetuses in regional centers around Boston, Massachusetts; Philadelphia, Pennsylvania; Toronto, Ontario; and San Diego, California; as well as birth defect records in Massachusetts and New York State. Mothers of un malformed babies (control) were identified in the participating birth hospitals and a population sample of un malformed babies in Massachusetts. Between January 1993 and June 1998, mothers were personally interviewed of 3,723 cases and 781 controls ( " phase 1 " ), and between August 1998 and January 2010 mothers were interviewed by telephone of 12,746 cases and 6,826 controls ( " phase 2 " ). In general, among mothers of the eligible cases and controls that could be contacted, the proportions that completed the interview were 69 per cent and 67 per cent respectively (). The study was approved by the institutional review boards of the relevant institutions and complies with the requirements of the Medical Insurance Portability and Responsibility Act. The cases that meet the requirements for the final analysis consisted of 12,734 infants and fetuses with a confirmed diagnosis of major congenital malformations, identified up to a minimum of 3 and a maximum of 5 months after delivery. Babies with chromosomal defects, known Mendelian inherited disorders, syndromes, DiGeorge sequence (associated with 22q elimination), and defects associated with prematurity were excluded. Cases with multiple anomalies were considered in each appropriate defect category. The controls that could be used for the final analysis consisted of 7,606 un malformed babies. Checking the Exposure Subjects who provided informed consent for the interview were asked to provide a release for their baby's medical records. Using highly structured interview procedures and questionnaires, trained study nurses interviewed mothers within 6 months of delivery. Information was requested on demographic characteristics; reproductive, medical and lifestyle factors; diet; and details of exposure to all medicines (prescription and OTC) at any time from 56 days prior to the date of the last menstrual period until the end of the pregnancy. Mothers and study nurses were not aware of the various hypotheses under consideration. To maximize memory of drug exposures and minimize errors in reported exposures, highly structured questionnaires included a series of more and more detailed questions (). The interviewers first asked about the occurrence of one of a list of diseases (e.g. infections) during pregnancy and the medications taken for these diseases, then about the use of categories of medicines (e.g. antibiotics, nasal sprays), and finally about the use of specific products, including brand and generic names. Mothers were asked to take a medicine that identified the dates on which the use began and ended; the memory was improved by a calendar that highlighted the key dates and events (e.g., last menstrual period, Christmas, delivery date). Subjects were also asked about the dose and form of the medication and the frequency and duration of use. A drug identification brochure was used with photographs of specific OTC drugs to improve the memory of TBT products. All medicines were encoded through the Slone Drug Dictionary, which links each product to its individual active ingredients. Changes in product formulations over time are incorporated into the Drug Dictionary, and new formulations are encoded accordingly. Definition and Classification of Exposure The date of the last menstrual period was based on early ultrasound examination or maternal memory. We define the estimated date of conception as 14 days after the date of the last menstrual period, the first trimester as 90 days after the estimated date of conception (composing the etiologically important period of structural development for most organs), the second trimester as 90 days after the first trimester, and the third trimester as the period after the second trimester through the end of pregnancy. We believe that exposure in any given quarter includes the mother's use of a decongestant at least 1 day. Using our exposure classification algorithm (), which considers uncertainty at the time of exposure of drugs based on the self-reported accuracy of the subject of start/stop dates and duration of use, we classify the exposure uncongested in the following categories: "not exposed at any time from 56 days before the last menstruation dated to the end of the pregnancy"; "simitablely exposed" and "possible" The reference group for all analyses was not exposed to any decongestant. Statistical Analysis We evaluate the specific defects that were reported previously to be associated with 1 or more decongestants: eye defects, hearing defects, septal defect ventricle, aorta coartation, endocardio defect, pyloric stenosis, atresia/small-intestinal stenosis, club foot, gastrosquisis and hemifacial microsomia. In addition, for 21 case groups that included at least 100 subjects each, we explore possible risks for other common specific defects. It is estimated that there are differences of separate probability and 95% confidence intervals for each specific birth defect in relation to each specific multivariate conditional logistical regression decongestantation, with the calendar year of interviews and geographical region of the birth hospital as the matching factors to control the effects of secular tendencies and regional variations in the recruitment of subjects of study and exhibitions. First-quarter anti-inflammatory numbers; age; premature weight and body mass index; educational level; alcohol consumption; diabetes; hypertension; asthma; allergy; first-quarter episodes of seizures, respiratory infection and fever; first trimester of use of acetaminophen, aspirin, ibuprofen, nonaspirin anti-Rhobial inflamatories in general; We adjust for possible congestions. For our exploratory analysis, we do not estimate the probabilities ratios of exposure with less than 5 cases or controls exposed due to the instability of such estimates. Because pseudoephedrine is combined with acetaminophen, and because an earlier study that had hypothesized an association with gastroschisis and atresia/stenosis small-intestinal () found the greatest risk for the combination of pseudoephedrine and acetaminophen, seudoephedrine exposure was stratified as if it was taken as a single-component product or combined. To evaluate the sensitivity of the results at the time of the exhibition, we perform separate analysis for exhibitions of second and third trimester, restricted to subjects who had not been exposed to the specific decongestant in the first trimester. All analyses were performed in SAS for Microsoft Windows, version 9.2 (SAS Institute Inc., Cary, North Carolina). RESULTSAmong non- malformed infant mothers (n = 7.606), 8.6 per cent of subjects (n = 656) reported taking 1 or more oral or intranasal decongestants during the first quarter. The use of oral products was reported by 7.8% of the subjects (n = 592); 7.0% (n = 531) only took pseudoephedrine, 0.4% (n = 28) phenylene only, and 0.4% (n = 30) phenylpropanolamine only. The use of intranasal decongestants was reported by 1.2% of the subjects (n = 92); oxymetazoline was the most common (0.9%, n = 66). The factors associated with the decongesting use of the first quarter are presented in the table. Table 1.Maternal characteristics selected in relation to the use of first aid of non-formal children, Slone Study Center for Epidemiology of Birth Defects, 1993–2010FeatureNonusersa (n=5.594)Usersb (n = 656)Crude Matched ORc95% CINo.%No.% Race/Eternal Ethnicity White non-Hispanicspanicspanics3,85468.9560 Black non-Hispanic4738.5253.80.30.2, 0.5 Non-Hispanic Asian, Pacific Islander3185.7132.00.30.2, 0.5 Other1272.391.40.50.3, 1.1 Maternal age, years Abbreviations: CI, confidence interval; OR, odds ratio.a Not exposed to any decongestant at any time from 56 days before the date of the last menstrual period until the end of the pregnancy.b Like any decongestant during the first trimester of pregnancy.c He coinced with the calendar year of interviews and the geographical region of the birth hospital. Probation percentages are not calculated for less than 5 users decongesting.d Not available due to lack of data.e The reference category for each variable is "no one during pregnancy", except for diabetes and hypertension, where the reference is "never".f The cough drugs included antitussives (dextromethorfa, benzonatate), expectorants (guaifenesin, guaiacolsulfonate potassium, ammonium carbonate, iodinated gliccerol), and mucolytics (acetylcysteine, carbocysteine).g Any exposure of 1 month before 1 month after the estimated date of conception. Oral Dismantants Evaluation of hypothesized associations For pseudoephedrine and ventricular septal defect (Table) the risk of unicomponent pseudoephedrine was approximating the null (Dds ratio (OR) = 1.1, 95% confidence interval (CI): 0.8, 95%), but the use of pseudoephedrine/acetaminophen was associated with a greater risk (OR = 1.87). Acetaminophen did not affect the risk (OR = 1.0, 95% CI: 0.9, 1.2). For phenylephrine, we observed a high risk for the defect of endocardial cushion (OR = 8.0, 95% CI: 2.5, 25.3; 4 cases exposed). The 4 cases exposed had multiple abnormalities, including other cardiovascular defects, and 1 had a member defect. For phenylpropanolamine, we find greater risks of hearing defects (OR = 7.8; 95% CI: 2.2, 27.2; 4 exposed cases) and piloeric stenosis (OR = 3.2, 95% CI: 1.1, 8.8; 6 exposed cases). None of the above-mentioned associations were observed by a second or third quarter (). Table 2.Odds Ratios and 95% of confidence interventions for the first trimester exposure to oral decreasing in relation to specific birth defects previously reported to be associated with the use of oral denganche, Slone Epidemiology Center Study of birth defects, 1993-2010 Outcome (Insolate and Multiple combined defects)bTotal No. 0.751 0.781 0.401 0.102 0.00783 0.078 0.078 0.078 0.024 0.078 0.024 0.078 0.024 0.078 0.078 0.078 0.078 0.078 0.078 0.00.078 0.078 0.0021 0.078 CI, confidence interval; OR, odds ratio.a Referring to the subjects exposed to be decongested in the first quarter (n = 1.833) between a total of 20,340 subjects.b Hemifacial microsomia was excluded from this table because the total number of cases (n = 47) was too small to allow for evaluation.c Not exposed to other decongestants in the first quarter.d All probability rates and confidence intervals of 95% were calculated on the conditional logistical regression basis equaled in the calendar year of interviews and geographical region of the birth hospital. In addition, for each exposure-outcome association, we adjust for factors associated with specific decongestant and the specific birth defect in our population that also materially changed the estimate of the probability ratio, as specified in the following footnotes. The probabilities ratios of less than 4 cases were not calculated. Adjusted for first-quarter episodes of respiratory infection and first-quarter use of cough.f Adjusted for first-quarter episodes of respiratory infection.g Adjusted for race/mother ethnicity.h Adapted for the maternal race/ethnicity and the use of first trimester of coughing medications.i Adjusted for alcohol consumption, first trimester episodes of respiratory infection and use of antihistamines.j Adjusted for the use of first trimesters of coughing.k Adjusted for the maternal race/ethnicity and educational level.l Restricted to non-gastroschisis cases, so as to exclude cases of small-intestinal atresia/stenosis, which can be secondary to gastroschisis.m Adjusted for the use of antihistamines.n Restricted to non-neural tube defect cases, so as to exclude cases of club, which can be secondary to a neural tube defect.o Adjusted for maternal age, weight of pregnancy, educational level and smoking. For pseudoephedrine and gastrosquisis (Table ), the use of pseudoephedrine of a single component was associated with a higher risk (OR = 3.0, 95% CI: 1.4, 6.2), while only 1 case of gastroschisis was exposed to pseudoephedrine/acetaminophen; on the contrary, for the combination of atresia/small-intestin, seudoephrine Acetaminophen was not associated with gastroschisis (OR = 0.9 95% CI: 0.7 1.3) or atresia/small intestinal sysosis (OR = 1.1, 95% CI: 0.8, 1.5). For hemifacial microsomia, the total number of cases (n = 47) was too small to allow the evaluation. Our findings did not support most of the previously hypothesized associations between first-quarter exposures to specific oral decongestants and specific defects; these include pseudoephedrine and coartation of the aorta (OR = 1.1, 95% CI: 0.7, 1.8), phenylephrine and eye defects and ears (only 1 case exposed for each group of exploratory defects) and clubs In our exploratory analysis of unprepared exposures and defects to be associated with decongestants, there were 84 comparisons of first-quarter exposure to oral decongestants in general, pseudoephedrine, phenylephrine and phenylpropanolamine in relation to another 21 specific groups of birth defects with at least 100 cases each. These analyses produced estimates of the adjusted probabilities ratio ranging from 0.7 to 2.4 (Table). Table 3 Analyses of the first-quarter exposure to Pseudoephedrineb in relation to specific birth defects It was not previously reported to be associated with the use of Decongestant, Slone Epidemiology Center Birth Defects Study, 1993–2010 2.1601.025.0.000. CI, confidence interval; OR, odds ratio.a It refers to topics discussed in the first quarter.b The results of the first-quarter exposure to phenylephrine only (n = 82) and phenylpropanolamine only (n = 57) are not presented in this table because there were less than 5 cases exposed for each specific birth defect, except for phenylephrine and cleft palate (adjusted OR = 1.7, 95% CI: 0.64.8; 5 cases exposed; first trimester). Not exposed to other decongestants in the first quarter.d All probability rates and confidence intervals of 95% were calculated on the conditional logistical regression basis equaled in the calendar year of interviews and geographical region of the birth hospital. In addition, for each exposure-outcome association, we adjust for factors associated with specific decongestant and the specific birth defect in our population that also materially changed the estimate of the probability ratio, as specified in the following footnotes. The probabilities ratios of less than 5 cases exposed for exploratory analysis were not calculated. Adapted for the weight of pregnancy, educational level and the use of cough drugs for the first time.f Adjusted for alcohol consumption, allergy and first-quarter episodes of respiratory infection.g Adjusted for maternal education level and allergy.h Adjusted for the use of cough medications.i Restricted to males only. Non-formal controls (restricted to men only): n = 3,768.j Adjusted for the use of first trimesters of acetaminophen and cough medications.k Adjusted for race/mother ethnicity and allergy.l Adjusted for maternal race/ethnicity and first trimester episodes of respiratory infection.m Adjusted for the use of antihistamines and cough medications. Only 1 of these comparisons had limits of confidence below 95% that exceeded 1.0: pseudoephedrine and defects of reduction of members (OR = 2,4, 95% CI: 1.5, 3,7) (Table ); this association was not observed for second or third trimester exposures (). The risk was raised both for the use of the first-quarter pseudoephedrine (OR = 2.5, 95% CI: 1.4, 4.5) and for the pseudoephedrine/acetaminophen (OR = 3.5, 95% CI: 1.7, 7.2). Acetaminophen did not affect the risk (OR = 1.1, 95% CI: 0.8, 1.6). Intranasal decongestants In our exploratory analysis of the first-quarter exposure to intranasal decongestants and previously unnotified defects associated with intranasal decongestant use, we made 150 comparisons that included intranasal decongestants in general, imidazoline derivatives in general, oxymetazoline, xylometazoline and non-imidaline derivatives The estimates of the adjusted probability ratio ranged from 0.7 to 3.3 (table ), with 95% confidence intervals for 2 specific malformations with first-quarter exposure, excluding the knot. Table 4.First quarter annalities: 0.502 intrasanosb waste relative to specific birth defects, Slone Epidemiology Center Birth Defects Study, 1993–2010cOutcome (Isolated and Multiple Defects Combined)Total No. 3,640.8 Bifida thorn, 3,641.1 cleft cleft with or without cleft cleft9791.41.40.7, 2.8111.11.70.8, 3.7 Tracheo-esofageal fistula25751.92.1g0.8, 5.551.93g1.2, 8 Referring to the subjects exposed to be decongested in the first quarter (n = 1.833) between a total of 20,340 subjects.b The results of the first-quarter exposure to non-imidazoline derivatives only (phenylphrine, levmetamphetamine, propylhexedrine) (n = 31) are not presented in this table because there are fewer than 5 cases exposed for each specific birth defect.c The results presented in this picture are only for specific birth defects with at least 5 cases exposed.d Not exposed to other decongestives in the first quarter.e All probability rates and confidence intervals of 95% were calculated on the conditional logistical regression basis equaled in the calendar year of interviews and geographical region of the birth hospital. In addition, for each exposure-outcome association, we adjust for factors associated with specific decongestant and the specific birth defect in our population that also materially changed the estimate of the probability ratio, as specified in the following footnotes. The odds ratios of less than 5 cases were not calculated for exploratory analysis.f Adjusted for race/mother ethnicity and educational level.g Adjusted for the maternal race/ethnicity.h Restricted to non-neural tube defect cases, so as to exclude cases of clubfoot, which can be secondary to a neural tube defect.i Adjusted for the maternal age; educational level; first-quarter episodes of respiratory infection; and first-quarter acetaminophen, antihistamines, cough medications and antibiotics.j Adapted for the level of education and the use of first trimester of coughing drugs.k Restricted to males only. Non-formal controls (restricted to males only): n = 3,768.Pyloric stenosis was associated with intranasal decongestants in general (OR = 1,9, 95% CI: 1.0, 3.5), imidazoline derivatives (oxymetazoline or xylometazoline) (OR = 2,2; 95% CI: 1.1, 4.5), and specifically xylometazoline (OR = 2,9) None of these associations were observed by a second or third quarter (). The risk of tracheoesophageal fistula was associated with the first-quarter exposure to imidazoline derivatives (OR = 3.3, 95% CI: 1.2, 8.9) (Table). This association was not observed by a second or third quarter (). We also find associations between renal collection system anomalies and second-quarter exposure to oxymetazoline (OR = 3.1, 95% CI: 1.3, 6.9) (). DISCUSSIONConsults of this case control study support previously hypothesized associations between the first trimester exposure to phenylene and endocardial damping defects (), between the first trimester exposure to phenylpropanolamine and ear defects (, ), and between the first trimester exposure to phenylpropanolamine and piloric stenosis (). In the exploratory analysis designed to generate hypothesis, multiple comparisons were identified between the use of pseudoephedrine and the defects of extremity reduction, between the use of intranasal decongestants and piloric stenosis, and between the use of the first trimester of imidazolin derivatives and the tracheoesofageal fistula, as well as the renal use of secondoxyazole For phenylephrine, the greatest risk of defect of endocardial cushion identified in a previous case control study (OR = 6.7, 90% CI: 1.3, 26; 2 cases exposed) () is replicated in our study (OR = 8.0, 95% CI: 2.5, 25.3; 4 cases exposed). It should be noted that fenilephrine belongs to the same class of fenulins replaced as phenfluramine and dexfenfluramine, which were associated with cardiac valvulopathy in adult users (). The 3 compounds share the chemical structure of 2-fenylethylamine. For phenylpropanolamine, the U.S. Collaborative Perinatal Project found a high risk of ocular and hearing defects (as a single group) (relative risk = 4.0, 95% CI: 1.6, 8.3) (), and a more recent study of the Swedish Registry identified a modest association for hearing defects (relative risk = 1.7, 95% CI: 0.8, 3.2) but not for ocular defects. We also replicate the association between pyloric stenosis and phenylpropanolamine (OR = 3.2, 95% CI: 1.1, 8.8; 6 cases exposed) reported in the same Swedish study (relative risk = 2.4, 95% CI: 0.9, 6.4; 4 cases exposed) (although the high risks we observed on the basis of relatively small numbers for the defect of the endocardion and the exposure of confusion, Control of factors other than those we included in our multivariate models did not substantially influence our results. As in all observational studies, retrospective information on maternal conditions in the first quarter (e.g. respiratory infection, fever) may be inaccurate, and residual confusion cannot be ruled out by uncontrolled factors. Previous research on the risks of gastroschisis by our group, including 1 study that included a subset of the cases reported here (), identified 1.8-fold () and 3.2-fold () probability ratios associated with any use of pseudoephedrine in early pregnancy. Our current analysis of women most likely to be exposed in early pregnancy provided weak support for such association, with a probabilities ratio of 1.5 times and a confidence interval of 95% lower than 0.8. However, when we separated the pseudoephedrine exposures into pseudoephedrine from a single component against pseudoephedrine/acetaminophen, the pattern of probabilities relationships was opposed to the previous study (): We found a high risk for gastroschisis with the use of pseudoephedrine of a single component (OR = 3.0, 95% CI: 1.4, 6.2), but only 1 case of gastrosquisis combined. Our findings for first-quarter pseudoephedrine and small-intestinal atresia/stenosis were compatible with an earlier report of our group (); in current analysis, we also observed a moderately greater risk for general use (OR = 1,6, 95% CI: 0.9, 2,8) that was greater for pseudoephedrine/acetaminophen (OR = 2,3, 95% CI: 0.96, no. Although a previous study found a risk of 2.4 times ventricular septal defect with first-quarter exposure to oral decongestants (95% CI: 0.9, 6.3) (), we find a probability ratio of only 1.3 (with a 95% narrow CI: 1.1, 1.6) for exposure to oral decongestants in general (mainly pseudoephedrine). It should be noted that the risk of being elevated only with pseudoephedrine/acetaminophen (OR = 1,8, 95% CI: 1.2, 2,7) and not with a single component pseudoephedrine (OR = 1.1, 95% CI: 0,8, 1.5). This finding is markedly consistent with the differences observed earlier by our group both for gastroschisis and for small-intestinal atresia in relation to the use of pseudoephedrine/acetaminophen in comparison with pseudoephedrine or paracetaminophen alone (). In other comparisons, we find little support for the associations between the first-quarter exposure to sympathetics (predominantly pseudoephedrine) and the coartation of the aorta (), the first-quarter exposure to phenylpropanolamine and gastroschisis (), and the first-quarter exposure to phenysylenephrine and ocular and ear defects () and club (). The results of our exploratory analysis corroborated previous reports describing any association between the first trimester exposure to oral decongestants and neural tube defects (), hypospadias (), or inguinal hernia (). Nor was there any appreciable association between the first-quarter oral decongestionative exposure and other specific birth defects, except for an increase of 2.4 times the risk of defects of reduction of members with pseudoephedrine. Understanding the risks associated with fenilephrine is important given recent trends in use (Figure ). Although pseudoephedrine remains the most commonly used decongestance, the relative use of phenylene increased after federal restrictions on access to pseudoephedrine (), which also led to the replacement of seudoephedrine with phenylene in some cough preparations. The use of phenylpropanolamine has decreased as a result of its market elimination in 2000–2001, which was due to its risk of hemorrhagic stroke in adults (). Secular trends in the use of any first quarter, as well as specific, decongestants among mothers of un malformed babies from Boston, Massachusetts and Philadelphia, Pennsylvania, who throughout the study period provided data to the study Slone Epidemiology Center Birth Defects Study, 1993-2010. The period 2009-2010 is essentially only 1 year because this study included data until January 2010. We believe that ours is the first study to explore the risks and relative safety of specific intranasal decongestives used during pregnancy with respect to a wide range of specific birth defects. Since the analysis of intranasal decongestants was of an exploratory nature, the associations we found justify a cautious interpretation because they were identified in the context of non-pre-existent hypothesis and multiple comparisons; the latter could result in confidence intervals that could be wider than we estimated. However, we believe that the observed high risks of 2 malformations justify a deeper exploration: pyloric stenosis in relation to the first-quarter exposure to intranasal decongestants, and traumatic fistula in relation to the first-quarter exposure to imidazoline derivatives. In addition, renal collection system abnormalities could be associated with the second-quarter exposure to oxymetazoline. It is not known if any of the oral decongestants (pseudoephedrine, phenylene and phenylpropanolamine) cross the placenta () to exercise any direct effect on the fetus. However, its vasoconstrictive properties as a result of α-adrenergic stimulation could cause constriction of uterine vessels that appear to have only α-adrenergic receptors (), potentially contributing to the risk of teratogenicity through vascular disturbance or oxidative stress induced by hypoxia (). Although it is administered intranasally for a intended localized effect and is generally thought to be less at risk than systemically administered agents, intranasal decongestants, mainly oxymetazoline and xylometazoline, are absorbed well from the nasal mucosa to produce systemic effects (). Although it is not known if these agents cross the placenta (), a report of changes in the fetal heart rate after the mother's use of oxymetazoline nasal spray () suggests that oxymetazoline and possibly other intranasal decongestants might affect the fetus. In addition to its agonistic vasoconstrictive α-adrenoceptor (), oxymetazoline and xylometazoline are also agonists of some subtypes of 5-hydroxytriptamine (serotonin) receptors (, ). Increased stimulation of 5-hydroxytetamine receptors could potentially affect fetal development and cause birth defects through 5-hydroxytoxytoxythaltic tetintotamines In its collection of drug stories, the BDS specifically asks about the use of OTC products, providing the opportunity to study this type of exposure to medicines. These analyses are often not possible with other types of data sources, such as claims or pharmacy dispensing databases, which do not usually capture OTC exposures. In addition, relying on the maternal report, the BDS focuses on the actual use of medicines (instead of prescription or dispensation). However, given that information on the use of drugs is obtained retrospectively, the potential of imperfect revocation is a concern, in particular the memory of TBT medications (such as decongestors) that tend to be used infrequent and short-lived ways. In the BDS, the use of a highly structured multi-level questionnaire () and a drug identification brochure with OTC drug photographs probably improved the memory of drug exposures. In addition, detailed information on the exposure of drugs collected by BDS allowed us to distinguish the subjects that were considered exposed (i.e., with high certainty of exposure) () from those that were possibly exposed. By creating separate categories instead of combining them in a single group of potentially exposed subjects, we seek to minimize the erroneous classification of the exhibition. We also focus on topics that had probably been exposed to a specific decongestant, without other decongestants in the same quarter; this allowed us to clearly distinguish the specific decongestants that could be associated with specific birth defects. Although this approach might have less power than others that add all potentially exposed subjects, we believe it offers greater validity by reducing the wrong classification of exposure. In our interview-based retrospective study, remembering the bias might be a concern if mothers of malformed babies remember or report the use of prenatal medicines differently to mothers of unformed controls. That we identify different effects estimates for different decongestants suggest that the recalled bias was of little relevance, because there is no reason to postulate that women would be biased in their memory of different decongestants in relation to different defects. Our findings should be kept in perspective. As an example, the prevalence of the defect of endocardial bearing is around 0.34 per 1,000 live births (); therefore, even if the exposure of phenylephrine increased the risk 8 times, the absolute risk of having an affected child would still be small (about 2.7 per 1,000 live births, or 0.27%). In short, our data supports some previously reported partnerships and raise a small number of new scenarios. Given the widespread use of decongestants by pregnant women, more data on the risks and relative safety of specific decongestants in relation to the wide range of specific birth defects remains to be obtained. Complementary materialNOWLEDGMENTAfiliations of authors: Department of Epidemiology, Harvard School of Public Health, Harvard University, Boston, Massachusetts (Wai-Ping Yau, Kueiyu Joshua Lin, Sonia Hernández-Díaz); Department of Pharmacy, Faculty of Sciences, Singapore National University, Singapore (Wai-Ping Yau); and Slone Epidemiology Center in Boston University, Boston, Massachusetts (Allen. This work was supported by R01 HD046595 of the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD). The Harvard School of Public Health Pharmacoepidemiology Program and the Slone Epidemiology Center are supported by several pharmaceutical companies, some of which could produce products included in these analyses. These analyses were not supported by any pharmaceutical manufacturer. Wai-Ping Yau was supported by the National University of Singapore-Overseas Postdoctoral Fellowship. Dawn Jacobs, Fiona Rice, Rita Krolak, Kathleen Sheehan, Moira Quinn, Clare Coughlin, Nancy Rodriquez-Sheridan, Carolina Meyers, Joan Shander and Paula Wilder for their assistance in data collection; Nastia Dynkin for computer programming; Massachusetts Department of Public Health staff for the Research and Prevention of Birth Defects, Dr. We also thank medical and nursing staff in all participating hospitals: Hospital de San Diego, California, California, California Conflict of interest: Until August 2012, Dr. Mitchell owned actions in Johnson and Johnson (REFERENCESFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
Afrin 0.05% Nasal Spray- Uses, Dosage, Side Effects, Price, Benefits, Online Pharmacy - DoctorOnCall
Afrin Nasal Spray - Uses, Active Ingredient, Dosage & Side Effects

Afrin Original Nasal Spray ‑ Shop Sinus & Allergy at H‑E‑B

22 Best Safe Nasal Sprays During Pregnancy: Reviews 2021
Tips Mom Pregnancy: Afrin Pregnancy
/pregnantcoldGettyImages-818131138-5a898f4da18d9e00370940b1.jpg)
Congestion and Nasal Symptoms During Pregnancy

22 Best Safe Nasal Sprays During Pregnancy: Reviews 2021
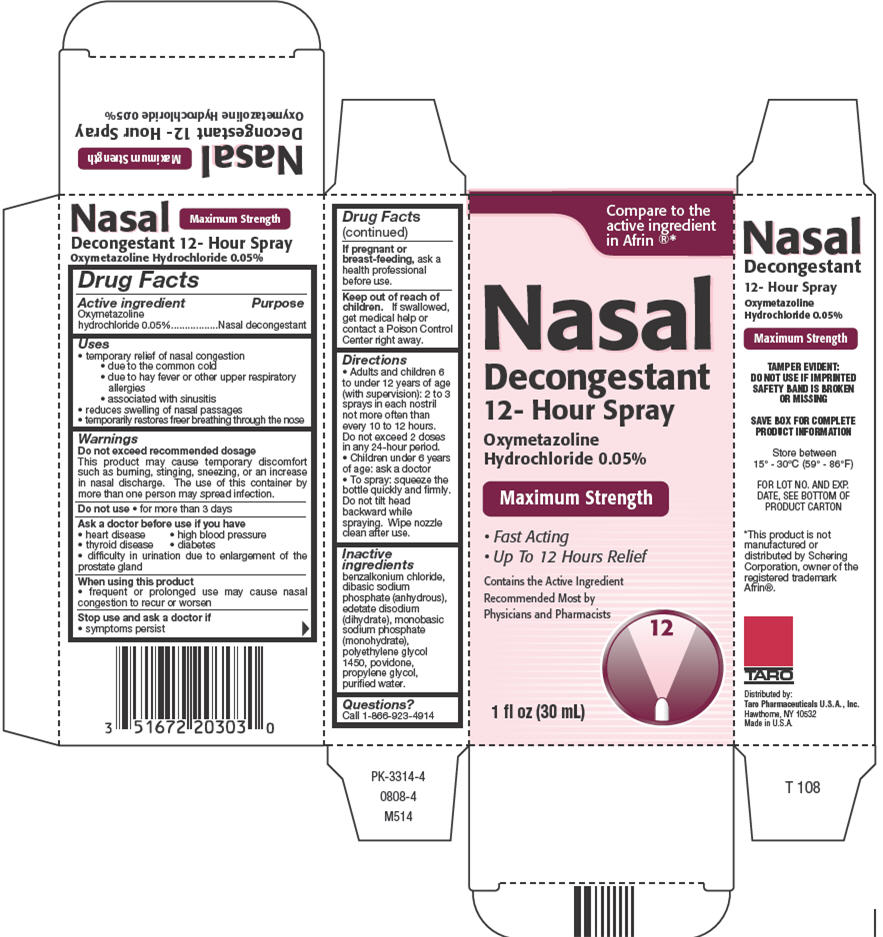
Oxymetazoline HydrochlorideNasal Decongestant 12-Hour Spray

Is it safe to use a nasal spray decongestant during pregnancy? | BabyCenter

Decongestant Nasal Spray During Pregnancy and Breastfeeding | babyMed.com
Afrin No Drip Severe Congestion Pump Mist ‑ Shop Sinus & Allergy at H‑E‑B
Afrin No Drip Severe 12 Hour Nasal Decongestant Pump Mist - 15 mL - Walmart.com - Walmart.com

Can You Use Nasal Spray During Pregnancy? That Congestion Is No Joke

Pin on baby information/pregnancy information

Nasal Sprays Safe for Pregnancy

Afrin vs. Flonase: Differences, similarities, and which is better for you
Afrin Nasal Spray 15ML - Medic Drugstore

Oxymetazoline Nasal Spray - Uses, Dose, Side effects, MOA, Brands |
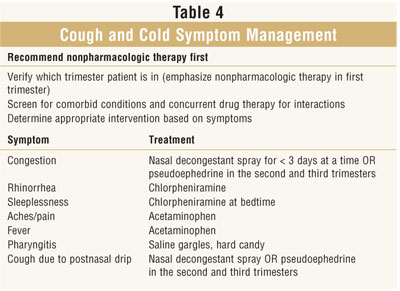
Pregnancy and OTC Cough, Cold, and Analgesic Preparations
Nasal Spray Recall Expands to Hundreds of Products

2 Pack) Equate Original No Drip Maximum Strength Oxymetazoline Nasal Spray, 1 Oz - Walmart.com - Walmart.com

Products to Help with Congestion During Pregnancy 2021

Nasal Sprays Work Best When You Use Them Correctly — Here's How – Health Essentials from Cleveland Clinic
SUDAFED® Nasal Decongestant Spray | Sudafed®

Amazon.com: Original Perrigo Original Nasal Spray 12 Hour Spray 1 Fl Oz. (30ml) Pack of Two: Health & Personal Care
ePharma.com.bd – Your Online Healthcare Solution

MUCINEX® SINUS-MAX® Nasal Spray - Clear & Cool 0.75 oz. | Mucinex® USA

AFRIN ORIGINAL DECONGESTANT NASAL SPRAY - CVS Pharmacy

Afrin Severe Congestion Nasal Spray | Walgreens

Equate Original Oxymetazoline Nasal Spray, 1oz | eBay

Treating Sinus Congestion While Pregnant | Ask Dr Sears
Oxymetazoline
Afrin Original Maximum Strength 12 Hour Nasal Congestion Relief Spray, 90 mL.
OVER THE COUNTER MEDICATIONS SAFE FOR USE IN PREGNANCY COMMON COLDS • Afrin® nasal spray • Saline spray • Robitussin® (s

Afrin Original Maximum Strength Nasal Congestion Relief Spray - 0.5 Fl Oz : Target

Drug Facts

Afrin 12 Hour Pump Mist, Original, 0.5 Ounce (3 Pack)
Iliadin Full Prescribing Information, Dosage & Side Effects | MIMS Singapore

Afrin Nasal Spray (Generic Oxymetazoline Nasal Spray) - Prescriptiongiant
:max_bytes(150000):strip_icc()/GettyImages-962829172-5bf3306dc9e77c002dd8159e.jpg)
How Afrin Nasal Spray Works as a Short-Term Decongestant
Posting Komentar untuk "afrin nasal spray pregnant"